Abstract
Background: Patients with organ dysfunction are often excluded from clinical trials. The results of most clinical trials are based on participants with normal organ function. In aggressive B cell lymphoma, hepatic and/or renal dysfunction may occur due to tissue infiltration with lymphoma, bulky lymphadenopathy leading to obstruction or tumor lysis leading to renal dysfunction. Some patients who develop aggressive B cell lymphoma may have preexisting renal and/or hepatic dysfunction. There is a paucity of data to guide therapy in patients with aggressive B cell lymphoma who present with hepatic and/or dysfunction. Therefore, we performed a retrospective single institution study in this population.
Methods: We retrospectively captured patient, disease, and treatment characteristics, as well as treatment response for consecutive patients with aggressive B cell lymphoma who presented with hepatic and/or renal dysfunction between November 2013 and July 2016 at the Levine Cancer Institute. Eligible patients were ≥ 18 years who received upfront chemoimmunotherapy and presented with hepatic dysfunction defined as serum AST or ALT > 3 x ULN or bilirubin > 1.5 x ULN and/or renal dysfunction defined as serum creatinine >2 or creatinine clearance < 40 ml/min prior to starting initial therapy. Dose adjustments to the individual chemotherapy drugs were captured. Response to therapy was assessed as per the Lugano criteria for response assessment in lymphoma. Patients with HIV or viral Hepatitis were included. Kaplan Meier techniques were utilized to estimate the survival distribution.
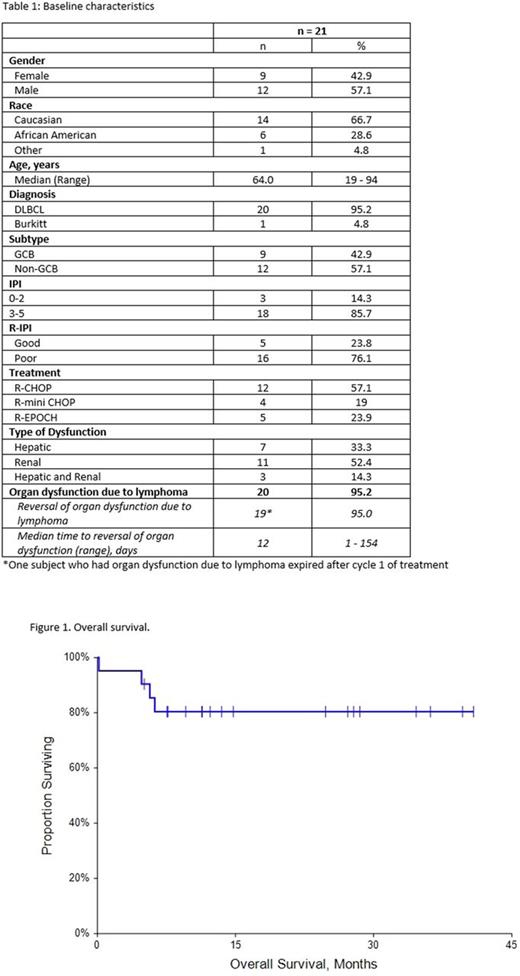
Results: Patient characteristics for the 21 cases are summarized in Table 1. Median follow up was 12.2 months (range 0.2 - 40.8 months). The majority of the patients were high risk with IPI score 3-5 in 18 patients (85.7%). Seventeen patients (81%) had advanced stage disease. R-CHOP or R mini CHOP was the upfront treatment for 76.2% of patients and 23.8% patients received dose adjusted R-EPOCH as their front line regimen. R-mini CHOP was used in patients who were > 80 years. Seven (33.3%) patients presented with hepatic dysfunction, 11 (52.4%) had renal dysfunction and 3 (14.3%) had both hepatic and renal dysfunction. Two patients had a prior history of chronic kidney disease and one patient had pre-existing hepatic dysfunction secondary to hepatitis C and cirrhosis. Four (19%) patients were pretreated with steroids prior to starting chemoimmunotherapy. During cycle 1, dose reductions due to organ dysfunction were performed in 5(23.8%) patients. Doxorubicin was dose reduced in 1 patient and held in 1 patient for > grade 3 hepatic dysfunction. Vincristine was dose reduced in 2 patients and held in 2 patients with > grade 3 hepatic dysfunction. Etoposide was dose reduced in 3 of the 5 patients who received R-EPOCH for either > grade 3 hepatic dysfunction or CrCl < 40ml/min. None of the patients received dose reductions in cyclophosphamide or rituximab. Median time to reversal of impaired organ function was 12.0 days. Nineteen patients (90.5%) were able to get full dose chemotherapy by cycle 2. One patient resumed full dose chemotherapy by cycle 3. One patient expired after cycle 1 of chemoimmunotherapy. Complete response was obtained in 18 patients (85.7%). The estimated 1-year overall survival rate was 80.4% (95% CI: 63.2-97.7%). Of the 4 patients who expired, one patient died after the first cycle of treatment, secondary to septic shock, one patient achieved a CR but was lost to follow up and expired from an unknown cause, and two patients had primary refractory disease and expired shortly after completing 6 cycles of chemoimmunotherapy. Despite the fact that the majority of the patients in our cohort had high risk disease, the efficacy results are comparable with chemoimmunotherapy in patients with aggressive B cell lymphoma who present with normal hepatic and renal function and met criteria for prospective clinical trials [1, 2].
Conclusion: Patients with hepatic and/or renal dysfunction usually present with high risk disease. Standard chemoimmunotherapy with dose adjustments in selected patients can lead to excellent outcomes in patients with aggressive B cell lymphomas who present with hepatic and/or renal dysfunction. Organ dysfunction related to lymphoma can reverse rapidly with initial treatment. Prospective clinical trials should be performed in patients with hepatic and/or renal dysfunction to confirm the results.
Ghosh: Jassen: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding. Trivedi: Pharmacyclics: Consultancy, Honoraria. Park: Takeda: Research Funding; Seattle Genetics: Research Funding; Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Gilead: Speakers Bureau; Teva: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal